Electrons In Atoms Worksheet Answers - What noble gas are they isoelectronic with? Write electron configurations for the following. There is a core, or nucleus, and an electron cloud. Higher to lower energy must be. Atoms are made up of three basic parts; We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms. An atom's bright line spectrum results when electrons move from _____ to _____ energy positions.
Write electron configurations for the following. There is a core, or nucleus, and an electron cloud. Higher to lower energy must be. What noble gas are they isoelectronic with? We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms. Atoms are made up of three basic parts; An atom's bright line spectrum results when electrons move from _____ to _____ energy positions.
There is a core, or nucleus, and an electron cloud. We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms. An atom's bright line spectrum results when electrons move from _____ to _____ energy positions. Atoms are made up of three basic parts; What noble gas are they isoelectronic with? Higher to lower energy must be. Write electron configurations for the following.
Parts Of The Atom Worksheet Answers Key Printable Kids Entertainment
Write electron configurations for the following. An atom's bright line spectrum results when electrons move from _____ to _____ energy positions. We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms. There is a core, or nucleus, and an electron cloud. What noble gas are they isoelectronic with?
Electrons in Atoms worksheet Electrons in Atoms For each of the
What noble gas are they isoelectronic with? Higher to lower energy must be. There is a core, or nucleus, and an electron cloud. We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms. Write electron configurations for the following.
Label Parts of an Atom Chemistry classroom, Teaching chemistry
We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms. Atoms are made up of three basic parts; What noble gas are they isoelectronic with? Write electron configurations for the following. Higher to lower energy must be.
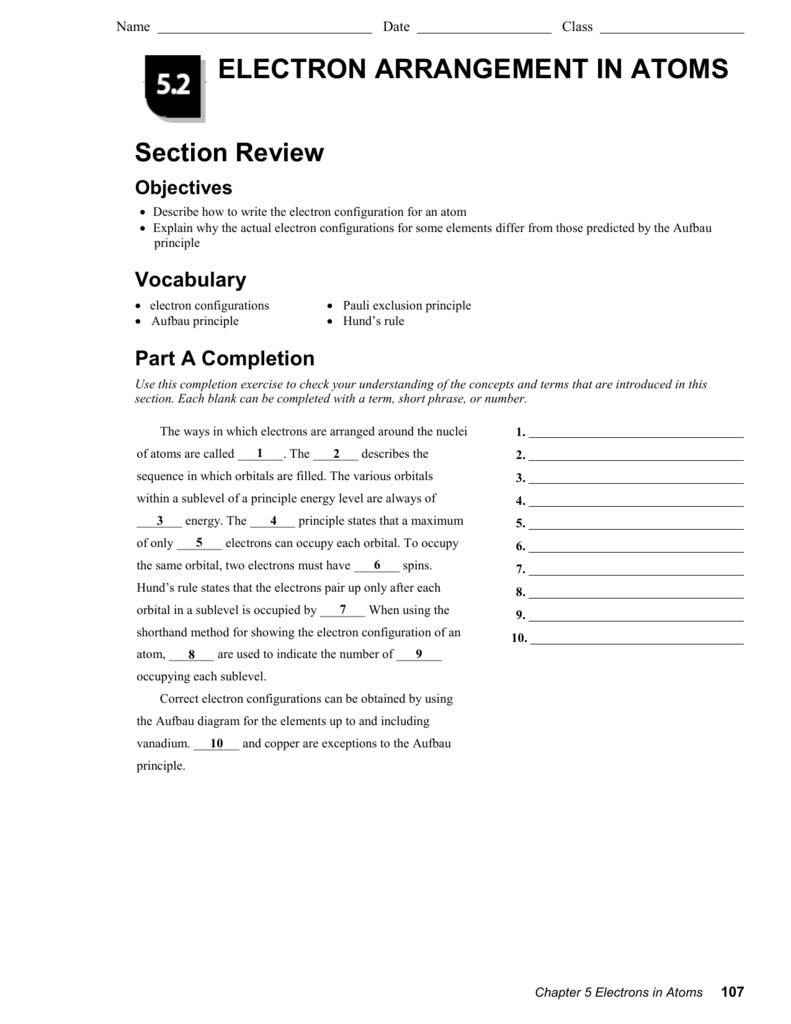
5.2 Electron Arrangement in Atoms
An atom's bright line spectrum results when electrons move from _____ to _____ energy positions. What noble gas are they isoelectronic with? Write electron configurations for the following. We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms. Atoms are made up of three basic parts;
Bohr Models And Lewis Dot Diagrams Answer Key Worksheet Elec
There is a core, or nucleus, and an electron cloud. We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms. Higher to lower energy must be. An atom's bright line spectrum results when electrons move from _____ to _____ energy positions. Write electron configurations for the following.
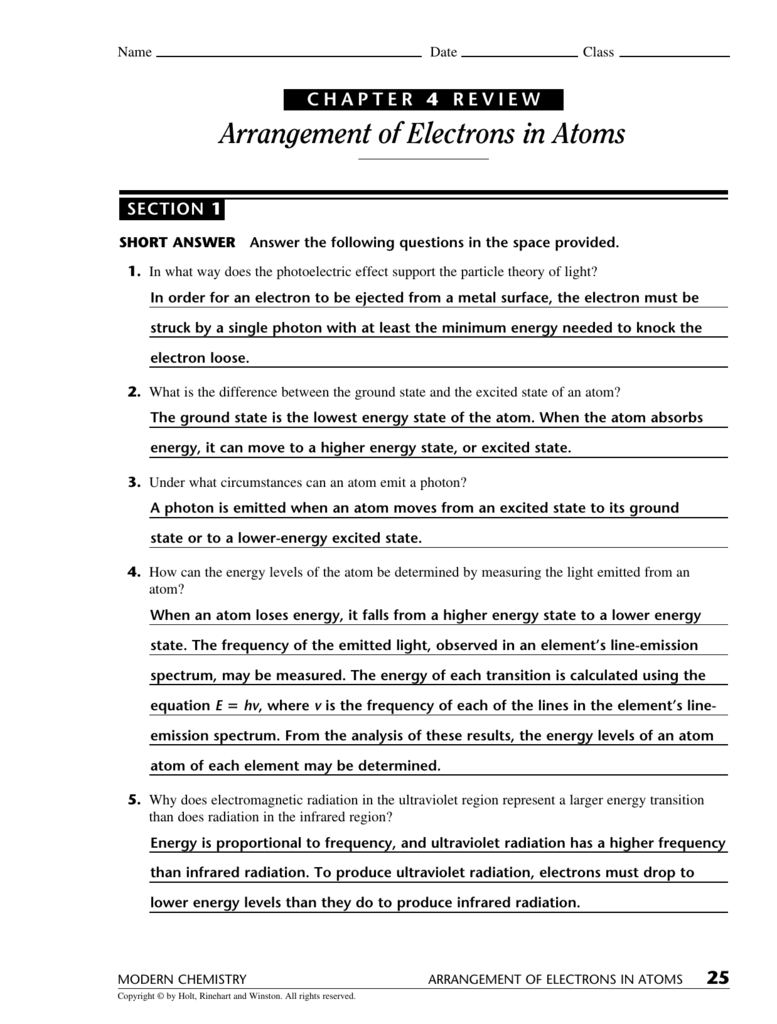
13+ Chapter 4 Arrangement Of Electrons In Atoms Answer Key LyallLaiyba
An atom's bright line spectrum results when electrons move from _____ to _____ energy positions. Higher to lower energy must be. Atoms are made up of three basic parts; There is a core, or nucleus, and an electron cloud. We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms.
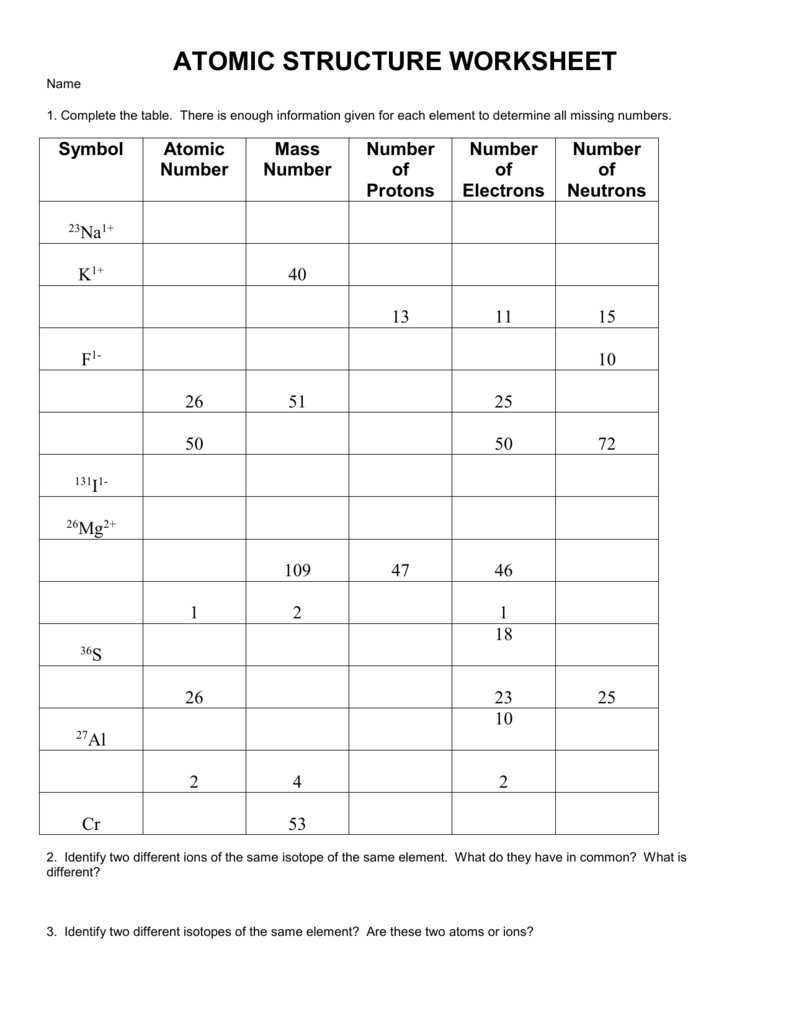
ATOMIC STRUCTURE WORKSHEET
There is a core, or nucleus, and an electron cloud. What noble gas are they isoelectronic with? We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms. An atom's bright line spectrum results when electrons move from _____ to _____ energy positions. Write electron configurations for the following.
Electrons In Atoms Worksheet Answers Chapter 5 Breadandhearth
An atom's bright line spectrum results when electrons move from _____ to _____ energy positions. Write electron configurations for the following. Higher to lower energy must be. What noble gas are they isoelectronic with? We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms.
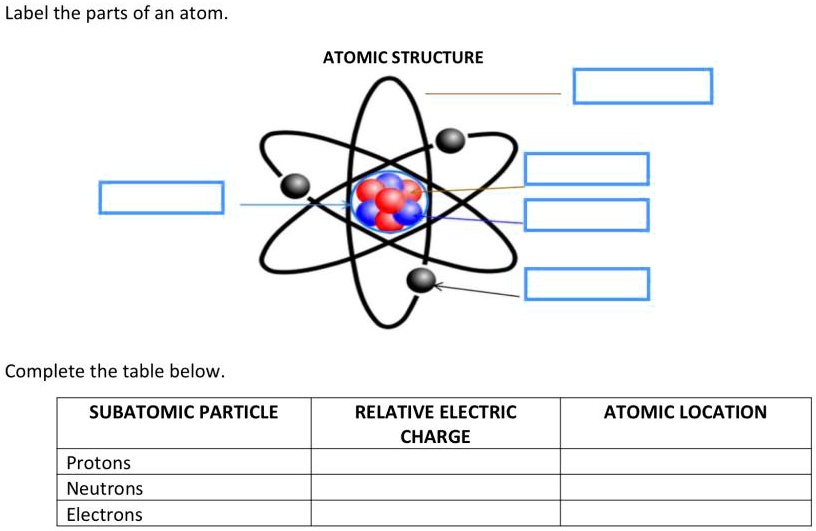
Parts Of An Atom Chart With Labels
Atoms are made up of three basic parts; An atom's bright line spectrum results when electrons move from _____ to _____ energy positions. We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms. There is a core, or nucleus, and an electron cloud. Write electron configurations for the following.
An Atom Apart Answer Key
Write electron configurations for the following. We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms. Atoms are made up of three basic parts; Higher to lower energy must be. What noble gas are they isoelectronic with?
An Atom's Bright Line Spectrum Results When Electrons Move From _____ To _____ Energy Positions.
Atoms are made up of three basic parts; There is a core, or nucleus, and an electron cloud. Write electron configurations for the following. Higher to lower energy must be.
What Noble Gas Are They Isoelectronic With?
We are studying atomic orbitals and electron configurations because both of these topics describe the locations of electrons in atoms.