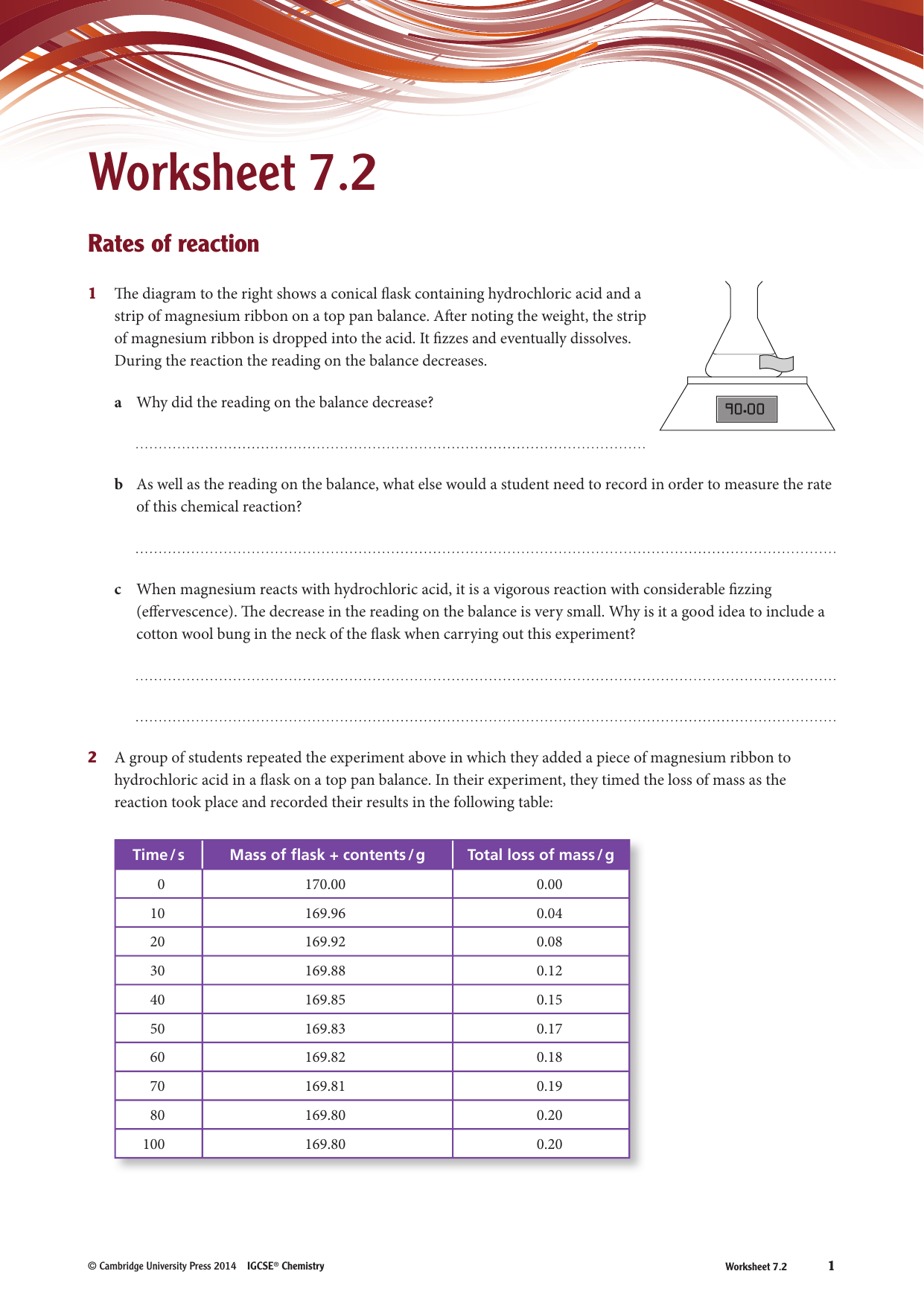
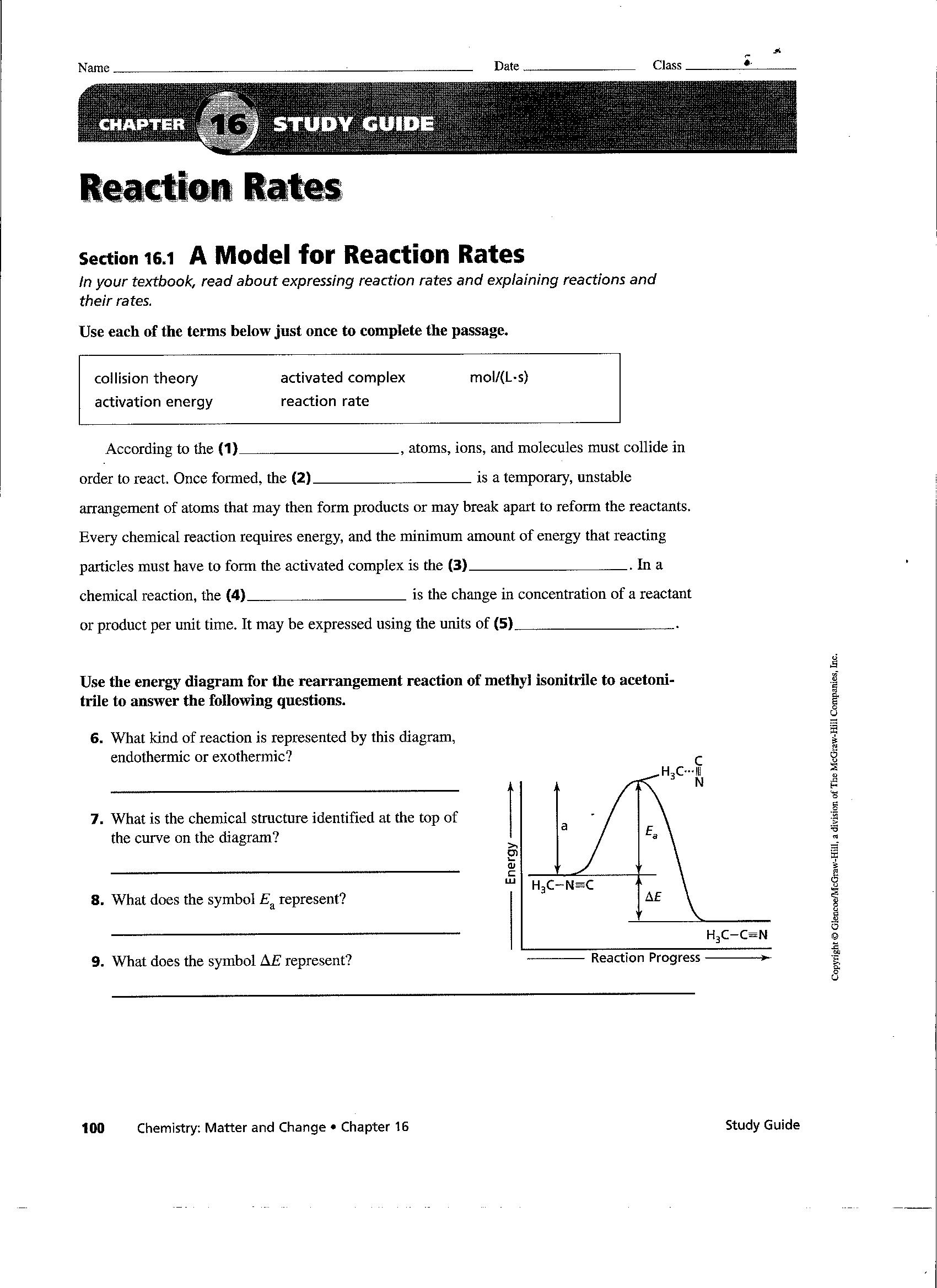
Factors Affecting The Rate Of Chemical Reactions Worksheet - What is the effect on rate of reaction when particle orientation is not important? (1) temperature, (2) particle size, and (3) concentration of the reactants. Why would a mixture of gases react faster when the volume they occupy is. What are the five major factors that affect reaction rate? How is reaction rate affected when particle. What factors affect the rate of a chemical reaction? Explain, using collision theory, what happens to the rate of the reaction as the reaction proceeds to completion. This worksheet explores the factors affecting the rate of chemical reactions. To summarise, the rate of reaction can be affected by: Initially, the concentration of hcl.
What are the five major factors that affect reaction rate? This worksheet explores the factors affecting the rate of chemical reactions. What is the effect on rate of reaction when particle orientation is not important? (1) temperature, (2) particle size, and (3) concentration of the reactants. Initially, the concentration of hcl. How is reaction rate affected when particle. To summarise, the rate of reaction can be affected by: Explain, using collision theory, what happens to the rate of the reaction as the reaction proceeds to completion. What factors affect the rate of a chemical reaction? The four main factors that affect the rate of chemical reactions are.
Why would a mixture of gases react faster when the volume they occupy is. This worksheet explores the factors affecting the rate of chemical reactions. What is the effect on rate of reaction when particle orientation is not important? The four main factors that affect the rate of chemical reactions are. (1) temperature, (2) particle size, and (3) concentration of the reactants. Initially, the concentration of hcl. What factors affect the rate of a chemical reaction? To summarise, the rate of reaction can be affected by: What are the five major factors that affect reaction rate? Explain, using collision theory, what happens to the rate of the reaction as the reaction proceeds to completion.
Rates of Reaction Worksheet 7.2
(1) temperature, (2) particle size, and (3) concentration of the reactants. How is reaction rate affected when particle. This worksheet explores the factors affecting the rate of chemical reactions. Why would a mixture of gases react faster when the volume they occupy is. To summarise, the rate of reaction can be affected by:
Factors Affectingh The Rate Of Chemical Reactions Worksheet
Why would a mixture of gases react faster when the volume they occupy is. Initially, the concentration of hcl. What is the effect on rate of reaction when particle orientation is not important? The four main factors that affect the rate of chemical reactions are. This worksheet explores the factors affecting the rate of chemical reactions.
SOLUTION Factors affecting the rate of reaction worksheet Studypool
This worksheet explores the factors affecting the rate of chemical reactions. What are the five major factors that affect reaction rate? Explain, using collision theory, what happens to the rate of the reaction as the reaction proceeds to completion. What is the effect on rate of reaction when particle orientation is not important? What factors affect the rate of a.
Factors Affecting the Rate of a Chemical Reaction
(1) temperature, (2) particle size, and (3) concentration of the reactants. Why would a mixture of gases react faster when the volume they occupy is. What factors affect the rate of a chemical reaction? What are the five major factors that affect reaction rate? How is reaction rate affected when particle.
Factors Affecting The Rate Of Chemical Reactions Worksheet Printable
The four main factors that affect the rate of chemical reactions are. Initially, the concentration of hcl. What is the effect on rate of reaction when particle orientation is not important? Why would a mixture of gases react faster when the volume they occupy is. (1) temperature, (2) particle size, and (3) concentration of the reactants.
Rate Of Chemical Reaction Worksheet
To summarise, the rate of reaction can be affected by: (1) temperature, (2) particle size, and (3) concentration of the reactants. What factors affect the rate of a chemical reaction? What are the five major factors that affect reaction rate? The four main factors that affect the rate of chemical reactions are.
Worksheet Factors Effecting Rates of Reaction
What is the effect on rate of reaction when particle orientation is not important? Why would a mixture of gases react faster when the volume they occupy is. This worksheet explores the factors affecting the rate of chemical reactions. What factors affect the rate of a chemical reaction? How is reaction rate affected when particle.
Factors Affecting The Rate Of Chemical Reactions Worksheet
This worksheet explores the factors affecting the rate of chemical reactions. What is the effect on rate of reaction when particle orientation is not important? What are the five major factors that affect reaction rate? How is reaction rate affected when particle. (1) temperature, (2) particle size, and (3) concentration of the reactants.
Gcse Reaction Rates Revise Factors Which Affect Them
Explain, using collision theory, what happens to the rate of the reaction as the reaction proceeds to completion. How is reaction rate affected when particle. To summarise, the rate of reaction can be affected by: What factors affect the rate of a chemical reaction? Why would a mixture of gases react faster when the volume they occupy is.
Worksheet 11 Measuring Reaction Rates
What are the five major factors that affect reaction rate? How is reaction rate affected when particle. (1) temperature, (2) particle size, and (3) concentration of the reactants. To summarise, the rate of reaction can be affected by: What factors affect the rate of a chemical reaction?
Explain, Using Collision Theory, What Happens To The Rate Of The Reaction As The Reaction Proceeds To Completion.
To summarise, the rate of reaction can be affected by: This worksheet explores the factors affecting the rate of chemical reactions. How is reaction rate affected when particle. Initially, the concentration of hcl.
What Factors Affect The Rate Of A Chemical Reaction?
Why would a mixture of gases react faster when the volume they occupy is. What is the effect on rate of reaction when particle orientation is not important? The four main factors that affect the rate of chemical reactions are. (1) temperature, (2) particle size, and (3) concentration of the reactants.