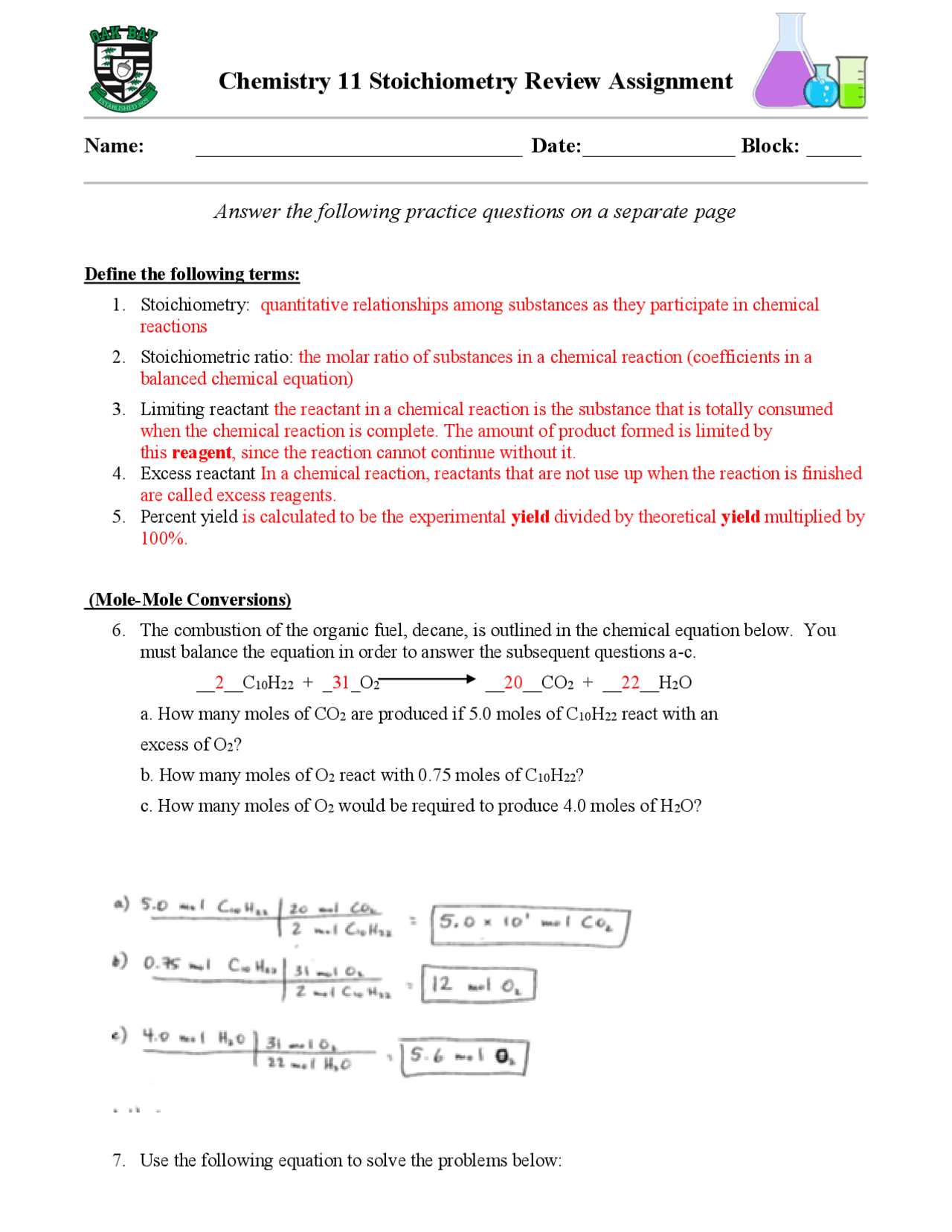
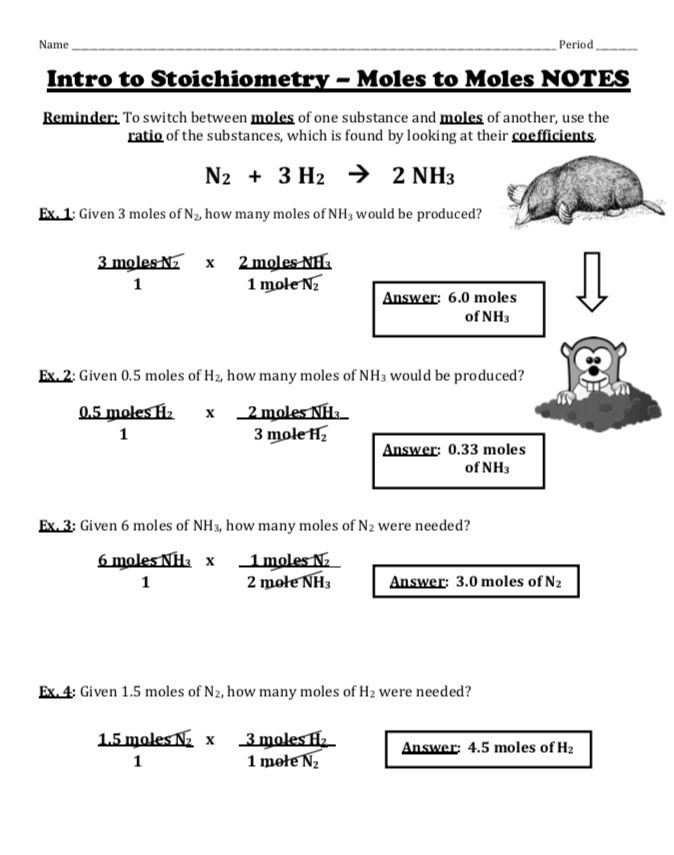
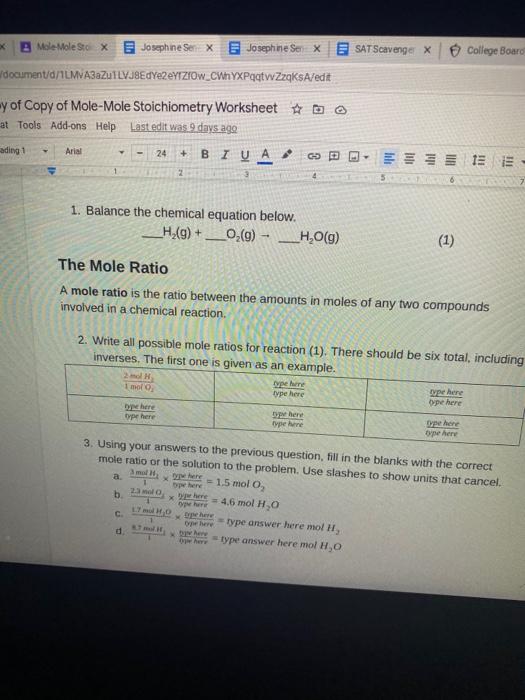
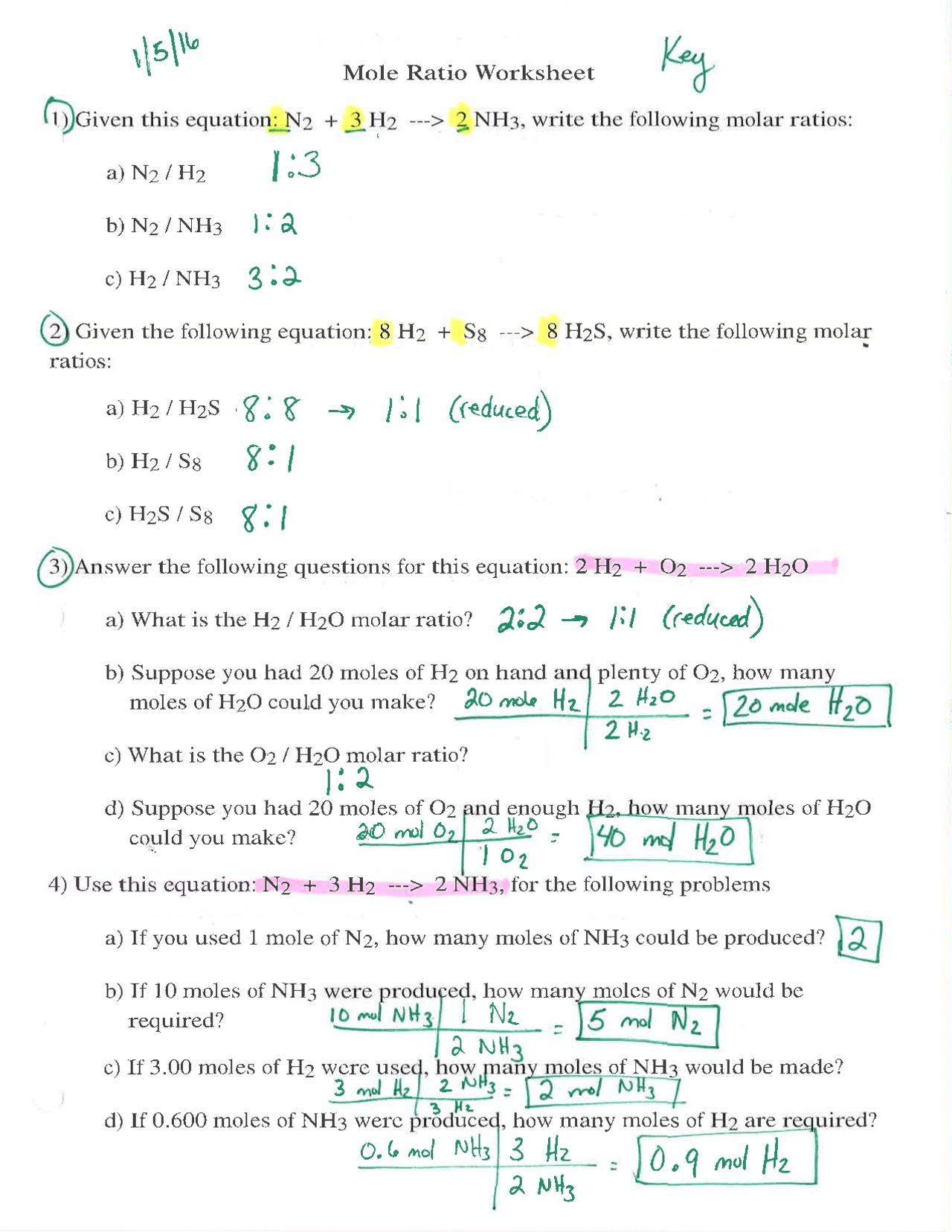
Mole To Mole Stoichiometry Worksheet - How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. The first page is a set of examples. Mole to mole problems 1. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
The first page is a set of examples. Mole to mole problems 1. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Mole to mole problems 1. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. The first page is a set of examples. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Worksheet Stoichiometry Mole to Mole Ratio with Answers Exercises Chemistry Docsity
Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. The first page is a set of examples. Mole to mole problems 1.
Moles & Stoichiometry WORKSHEET W1 Avogadro's Number
The first page is a set of examples. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?.
Catch Up Stoichiometry Worksheet With Answers PDF Mole (Unit Worksheets Library
Mole to mole problems 1. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. The first page is a set of examples.
INTRODUCTION TO THE MOLE RATIO & STOICHIOMETRY Worksheet
How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Mole to mole problems 1. The first page is a set of examples. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Mole to Mole Stoichiometry Detailed Examples and Practice Worksheets Library
Magnesium reacts with hydrochloric acid according to the following balanced chemical. The first page is a set of examples. Mole to mole problems 1. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?.
Mole To Mole Stoichiometry Calculations Worksheet
How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. The first page is a set of examples. Mole to mole problems 1.
of MoleMole Stoichiometry Worksheet Addons Help
Mole to mole problems 1. Magnesium reacts with hydrochloric acid according to the following balanced chemical. The first page is a set of examples. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?.
Mole Ratios Worksheet with Answers Chemistry Exercises Worksheets Library
Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1. The first page is a set of examples. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?.
Solved Stoichiometry Worksheet 1 MoletoMole and
How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. The first page is a set of examples. Mole to mole problems 1.
Mole To Mole Problems 1.
Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. The first page is a set of examples.