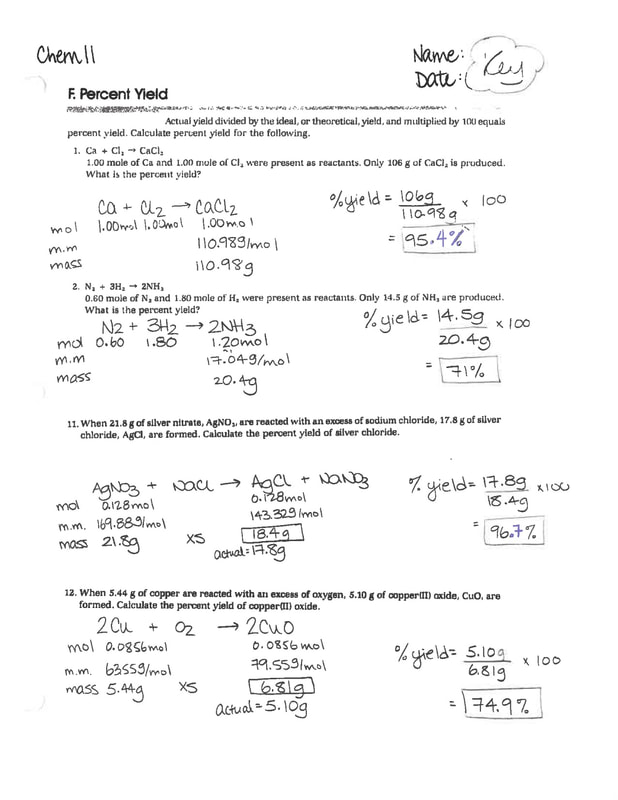
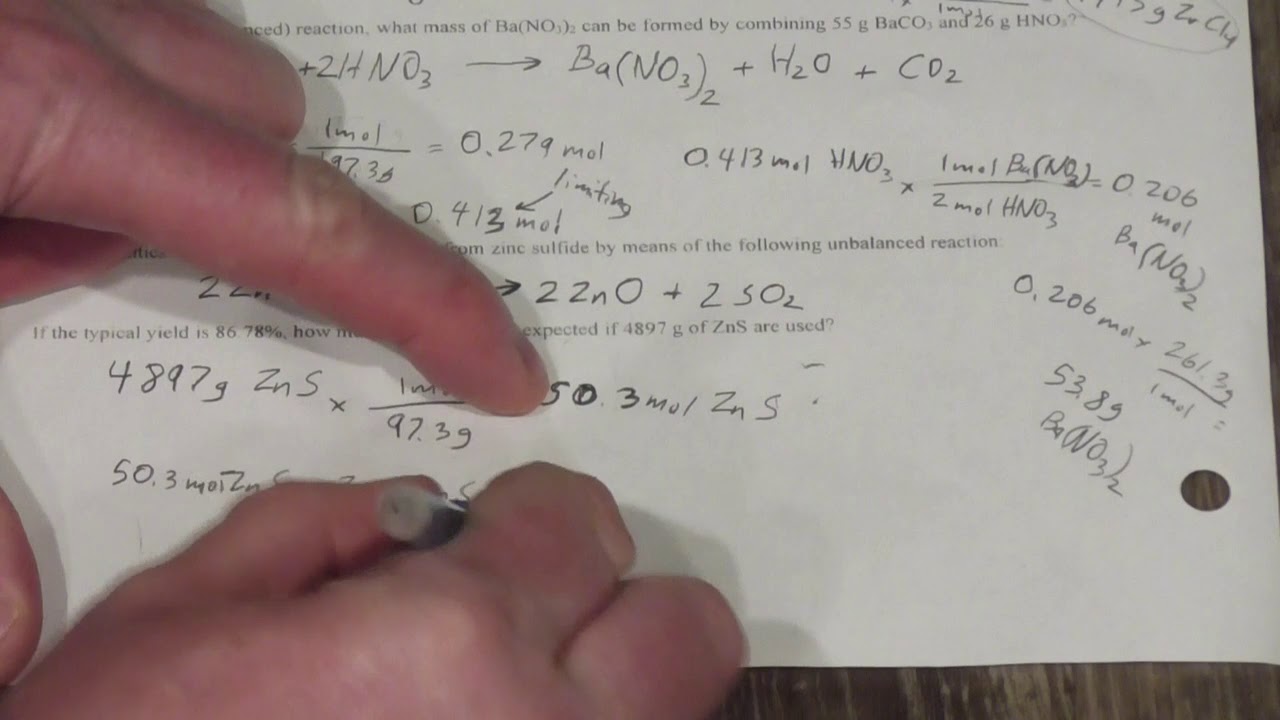Percent Yield Worksheet Answers - Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. To answer this, we just use the following. If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction?
1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. To answer this, we just use the following. If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g.
To answer this, we just use the following. 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g.
Chemistry Percent Yield Worksheet Answers
If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? To answer this, we just use the following. 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. Calculate the percent yield of a.
Percent Yield Stoichiometric Exercises Exercises Chemistry Docsity
Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? To answer this, we just use the following. 1) write a balanced equation for the.
Limiting Reagent And Percent Yield Worksheet Chemistry classroom
1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? To answer this, we just use the following. Calculate the percent yield of a.
Theoretical And Percent Yield Worksheet Answers worksheet
Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. To answer this, we just use the following. If 11.3 grams of sodium chloride are.
Percent Yield Worksheet Answers Chemistry A Study Of Matter
1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. To answer this, we just use the following. If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? Calculate the percent yield of a.
Limiting Reactant and Percent Yield Worksheet Side 2, 7 YouTube
If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? To answer this, we just use the following. Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. 1) write a balanced equation for the.
Limiting Reagent And Percent Yield Worksheet
1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. If 11.3 grams of sodium chloride are formed in the reaction described in problem #2,.
Limiting Reagents And Percentage Yield Worksheets Answers
To answer this, we just use the following. If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. Calculate the percent yield of a.
Limiting Reagent And Percent Yield Worksheet
1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? Calculate the percent yield of a reaction that had a theoretical yield of 3.76.
Percent Yield Worksheet Sulfate Chemical Reactions
If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. To answer this, we just use the following. 1) write a balanced equation for the.
To Answer This, We Just Use The Following.
1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. If 11.3 grams of sodium chloride are formed in the reaction described in problem #2, what is the percent yield of this reaction? Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g.