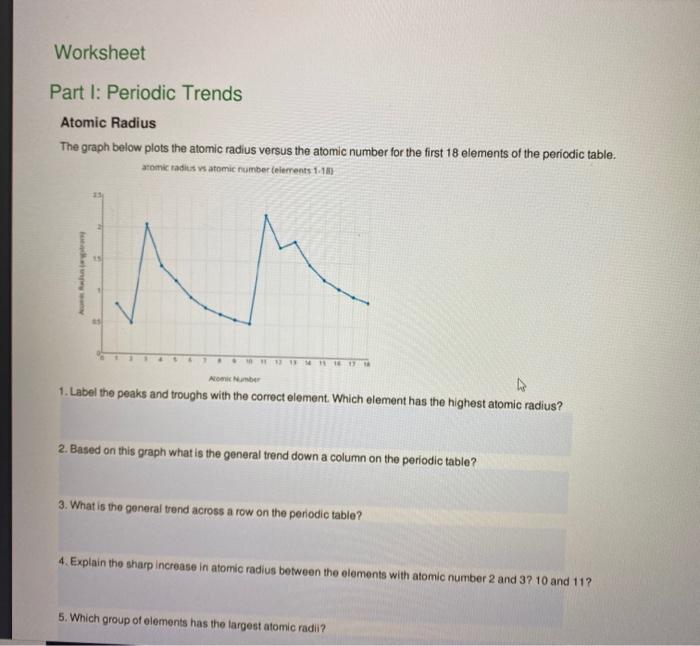
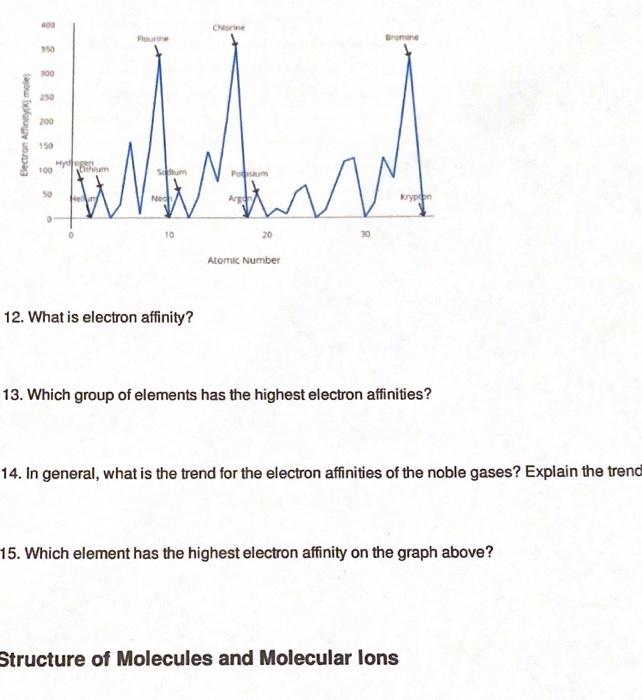
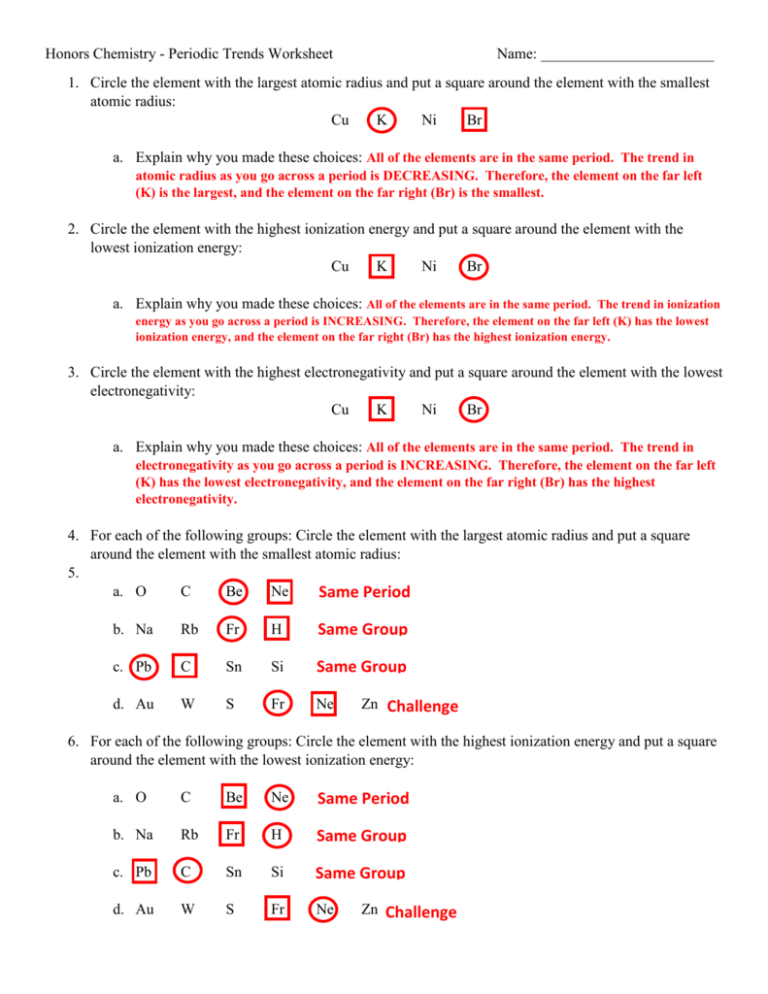
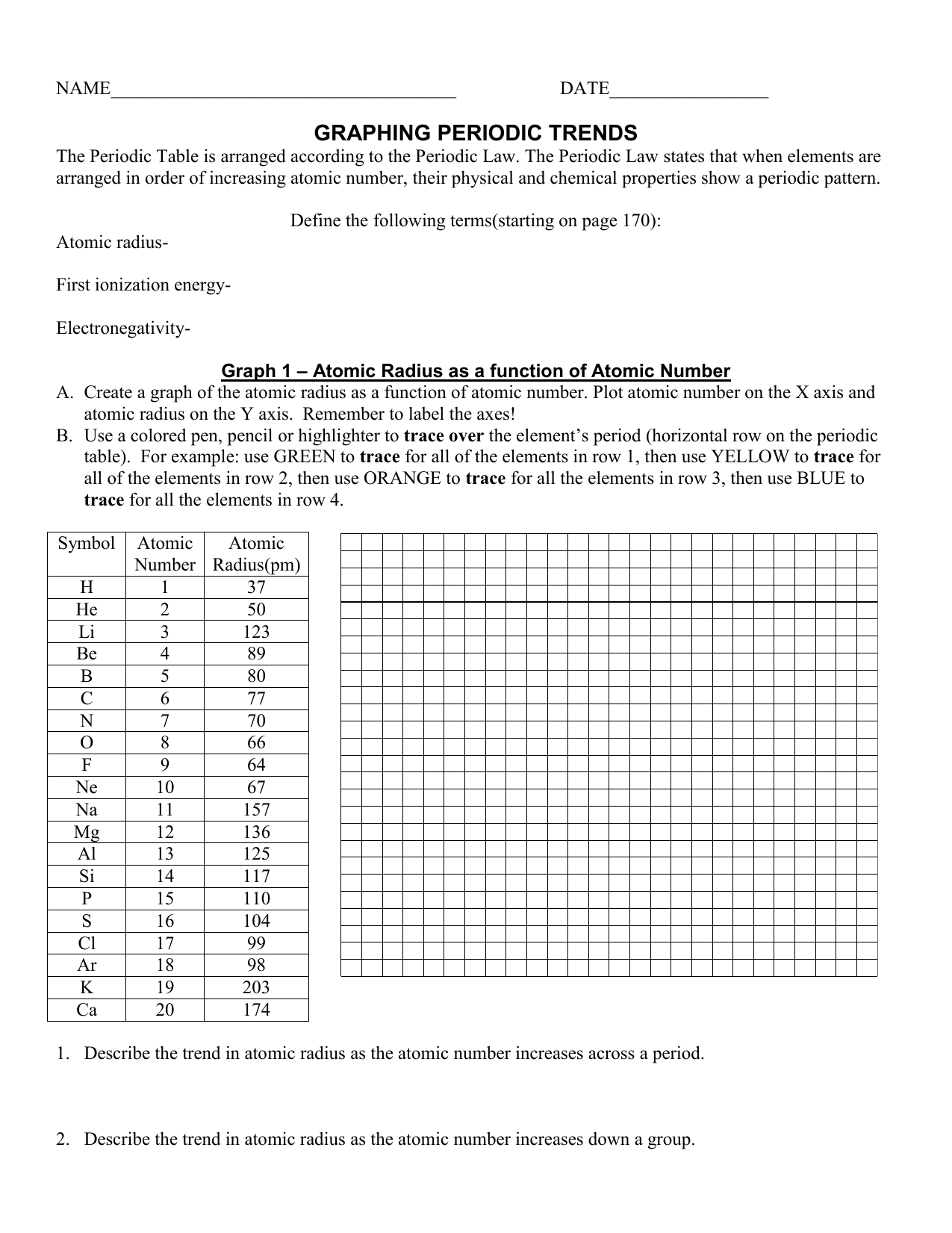
Periodic Trends Atomic Radius Worksheet Answers - Periodic trends tutorial 2 1. Use your notes to answer the following questions. For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Compare the effects of atomic radius on the first ionization energy of an atom. Draw arrow showing which directions atomic radius increases. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Which of the following in each pair has the larger atomic radius?
Periodic trends tutorial 2 1. Compare the effects of atomic radius on the first ionization energy of an atom. For each of the following sets of atoms, rank the atoms from smallest to largest atomic. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Which of the following in each pair has the larger atomic radius? Draw arrow showing which directions atomic radius increases. Use your notes to answer the following questions.
Draw arrow showing which directions atomic radius increases. Periodic trends tutorial 2 1. Compare the effects of atomic radius on the first ionization energy of an atom. Which of the following in each pair has the larger atomic radius? Use your notes to answer the following questions. For each of the following sets of atoms, rank the atoms from smallest to largest atomic. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___
Solved Worksheet Part I Periodic Trends Atomic Radius The
For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Compare the effects of atomic radius on the first ionization energy of an atom. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Periodic trends tutorial 2 1. Which of the following in each pair has the larger.
Solved Worksheet Part I Periodic Trends Atomic Radius The
Compare the effects of atomic radius on the first ionization energy of an atom. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Which of the following in each pair has the larger atomic radius? For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Draw arrow showing.
Periodic Table Atomic Radius Chem Worksheet 61 2024 Periodic Table
Compare the effects of atomic radius on the first ionization energy of an atom. Draw arrow showing which directions atomic radius increases. Which of the following in each pair has the larger atomic radius? Periodic trends tutorial 2 1. For each of the following sets of atoms, rank the atoms from smallest to largest atomic.
Periodic Trends Atomic Radius Worksheet Answers
Compare the effects of atomic radius on the first ionization energy of an atom. Draw arrow showing which directions atomic radius increases. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Periodic trends tutorial 2 1. Use your notes to answer the following questions.
Periodic Trends Worksheets With Answers
1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Compare the effects of atomic radius on the first ionization energy of an atom. Use your notes to answer the following questions. Draw arrow showing which directions atomic radius increases. Which of the following in each pair has the larger atomic radius?
Periodic Trends Worksheet Atomic Radius Which atom in each pair has
1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Compare the effects of atomic radius on the first ionization energy of an atom. Draw arrow showing which directions atomic radius increases. For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Which of the following in each pair.
Periodic Trends Worksheet Answers
Compare the effects of atomic radius on the first ionization energy of an atom. Periodic trends tutorial 2 1. Which of the following in each pair has the larger atomic radius? 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ Use your notes to answer the following questions.
Graphing Periodic Trends Worksheet Answers Printable Calendars AT A
Which of the following in each pair has the larger atomic radius? Draw arrow showing which directions atomic radius increases. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Compare the effects of atomic radius on the first.
Periodic Trends Worksheet Answers
Compare the effects of atomic radius on the first ionization energy of an atom. Which of the following in each pair has the larger atomic radius? Use your notes to answer the following questions. Periodic trends tutorial 2 1. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___
Periodic Trends Atomic Radius Worksheet Answers Printable Calendars
For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Which of the following in each pair has the larger atomic radius? Draw arrow showing which directions atomic radius increases. Periodic trends tutorial 2 1. Use your notes to answer the following questions.
Use Your Notes To Answer The Following Questions.
Periodic trends tutorial 2 1. 1) li or k ___k____ 2) ca or ni ___ca___ 3) ga or b ___ga___ For each of the following sets of atoms, rank the atoms from smallest to largest atomic. Draw arrow showing which directions atomic radius increases.
Compare The Effects Of Atomic Radius On The First Ionization Energy Of An Atom.
Which of the following in each pair has the larger atomic radius?