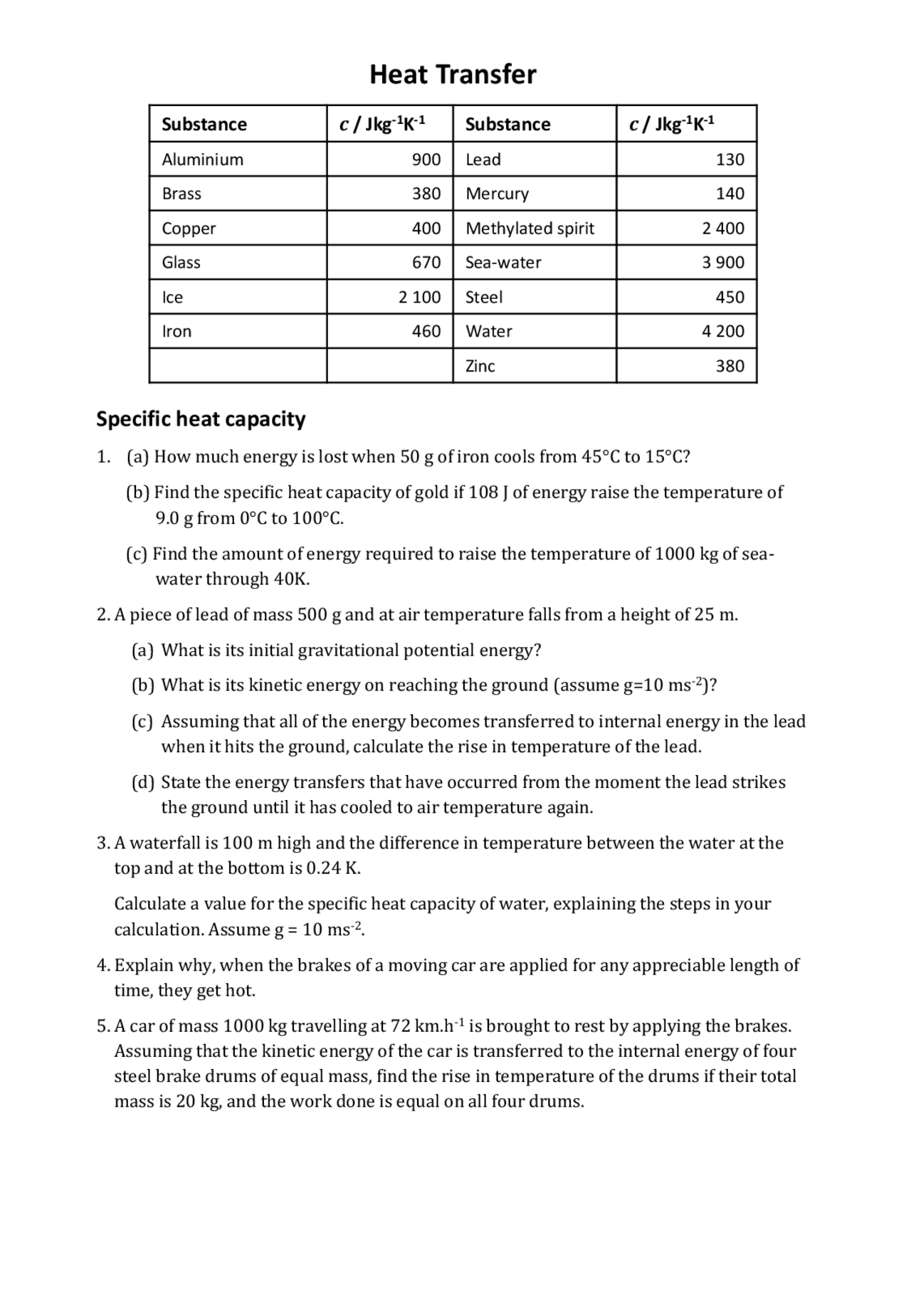
Specific Heat And Heat Capacity Worksheet Answers - Heat practice problems q = m x ∆t x c 1. How much energy was used to heat cu? Worksheet 12.2 latent heat, specific heat, and work 1. It requires more energy to raise its temperature by the same amount. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. What does it mean if water has a higher specific heat capacity than oil? Calculate the specific heat capacity of iron. 5.0 g of copper was heated from 20°c to 80°c.
Heat practice problems q = m x ∆t x c 1. It requires more energy to raise its temperature by the same amount. How much energy was used to heat cu? Worksheet 12.2 latent heat, specific heat, and work 1. The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. What does it mean if water has a higher specific heat capacity than oil? 5.0 g of copper was heated from 20°c to 80°c. Calculate the specific heat capacity of iron. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to.
Calculate the specific heat capacity of iron. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Worksheet 12.2 latent heat, specific heat, and work 1. 5.0 g of copper was heated from 20°c to 80°c. Heat practice problems q = m x ∆t x c 1. How much energy was used to heat cu? The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher specific heat capacity than oil?
Specific Heat And Heat Capacity Worksheet
Heat practice problems q = m x ∆t x c 1. Calculate the specific heat capacity of iron. Worksheet 12.2 latent heat, specific heat, and work 1. What does it mean if water has a higher specific heat capacity than oil? 5.0 g of copper was heated from 20°c to 80°c.
Specific Heat Worksheet Answers 1 Worksheets Library
How much energy was used to heat cu? The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. What does it mean if water has a higher specific heat capacity than oil? It requires more energy to raise its temperature by the same amount. Calculate.
11 Science Heat Energy Worksheets With Answer Heat energy
Calculate the specific heat capacity of iron. 5.0 g of copper was heated from 20°c to 80°c. It requires more energy to raise its temperature by the same amount. Heat practice problems q = m x ∆t x c 1. How much energy was used to heat cu?
30++ Specific Heat Worksheet Answers Worksheets Decoomo
Calculate the specific heat capacity of iron. Worksheet 12.2 latent heat, specific heat, and work 1. Heat practice problems q = m x ∆t x c 1. 5.0 g of copper was heated from 20°c to 80°c. What does it mean if water has a higher specific heat capacity than oil?
Solved Heat Transfer/ Specific Heat Problems Worksheet
Heat practice problems q = m x ∆t x c 1. The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. Worksheet 12.2 latent heat, specific heat, and work 1. How much energy was used to heat cu? Calculate the specific heat capacity of iron.
Solved Specific Heat and Heat Capacity Worksheet DIRECTIONS
How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Calculate the specific heat capacity of iron. Heat practice problems q = m x ∆t x c 1. The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one.
KS4 GCSE Physics Specific Heat Capacity Formula Worksheet with
5.0 g of copper was heated from 20°c to 80°c. It requires more energy to raise its temperature by the same amount. Heat practice problems q = m x ∆t x c 1. Worksheet 12.2 latent heat, specific heat, and work 1. Calculate the specific heat capacity of iron.
Heat Transfer Specific Heat Capacity and Specific Latent Heat Worksheet
How much energy was used to heat cu? It requires more energy to raise its temperature by the same amount. 5.0 g of copper was heated from 20°c to 80°c. Worksheet 12.2 latent heat, specific heat, and work 1. What does it mean if water has a higher specific heat capacity than oil?
42 specific heat capacity worksheet answers Worksheet Master
How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. 5.0 g of copper was heated from 20°c to 80°c. It requires more energy to raise its temperature by the same amount. Worksheet 12.2 latent heat, specific heat, and work 1. What does it mean if water has a higher specific.
Specific Heat Capacity Worksheet
How much energy was used to heat cu? 5.0 g of copper was heated from 20°c to 80°c. Calculate the specific heat capacity of iron. The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. It requires more energy to raise its temperature by the.
How Much Energy Was Used To Heat Cu?
The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. What does it mean if water has a higher specific heat capacity than oil? It requires more energy to raise its temperature by the same amount. Calculate the specific heat capacity of iron.
5.0 G Of Copper Was Heated From 20°C To 80°C.
Heat practice problems q = m x ∆t x c 1. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to. Worksheet 12.2 latent heat, specific heat, and work 1.