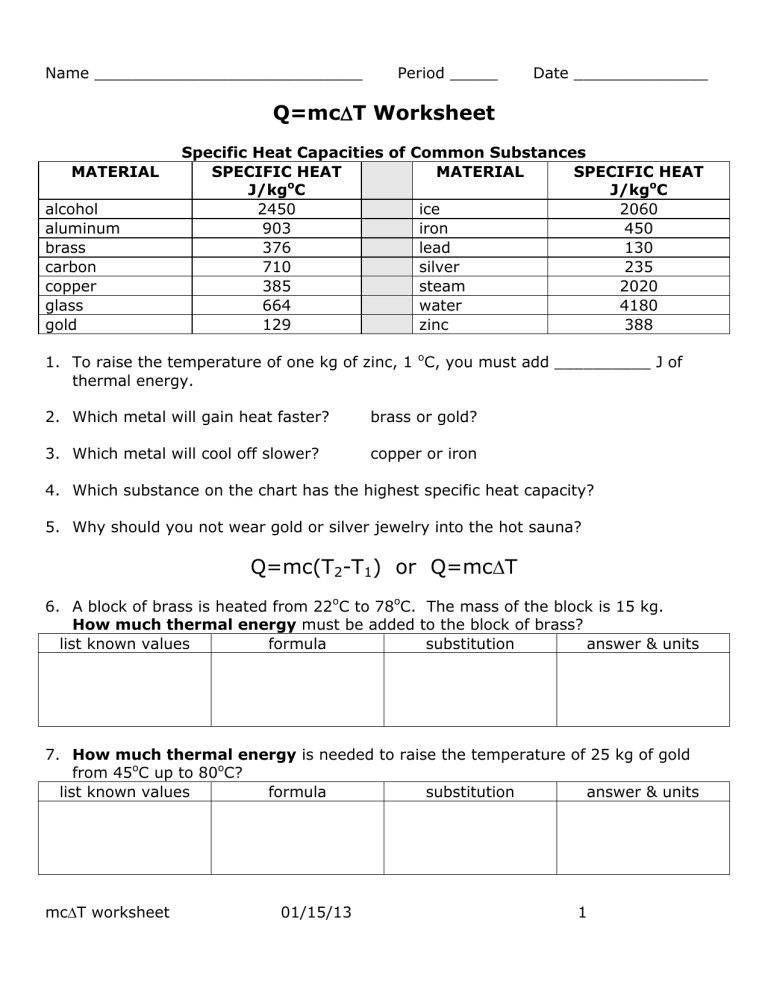
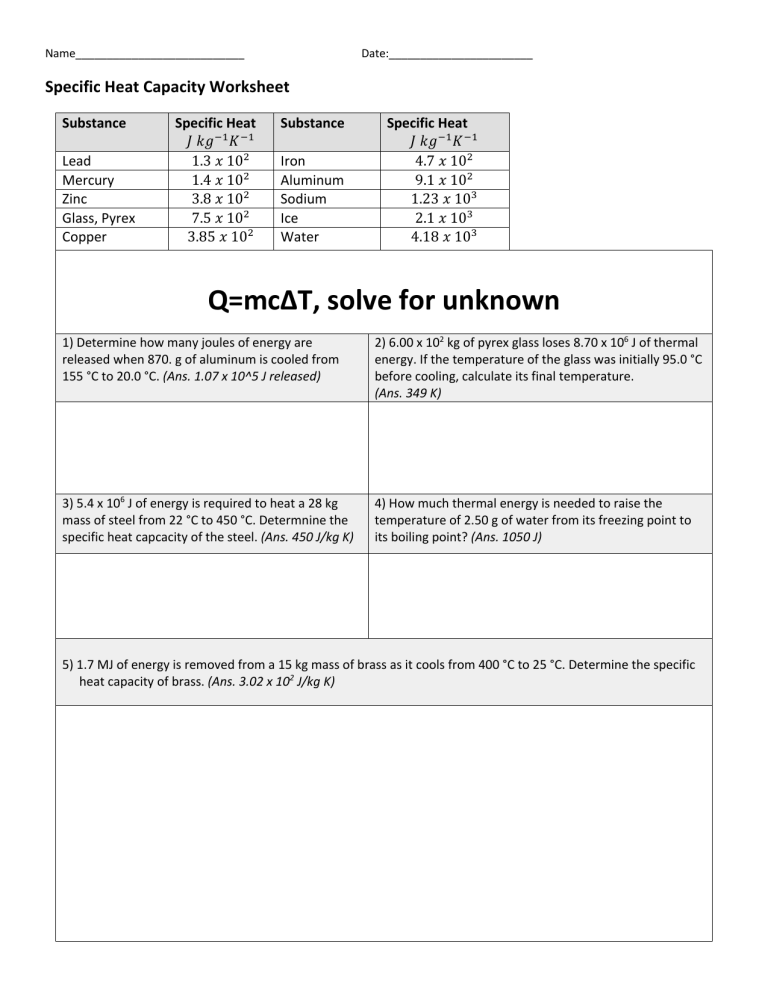
Worksheet Introduction To Specific Heat Capacities - A) the highest specific heat capacity? Based on the definition above, which of the 4 substances do you think has: Water (see < raph) b). The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree celsius (or. It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher specific heat capacity than oil?
Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree celsius (or. Water (see < raph) b). What does it mean if water has a higher specific heat capacity than oil? Based on the definition above, which of the 4 substances do you think has: A) the highest specific heat capacity? The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. It requires more energy to raise its temperature by the same amount.
Based on the definition above, which of the 4 substances do you think has: It requires more energy to raise its temperature by the same amount. Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree celsius (or. The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. A) the highest specific heat capacity? What does it mean if water has a higher specific heat capacity than oil? Water (see < raph) b).
Worksheet Introduction To Specific Heat Capacities Printable
What does it mean if water has a higher specific heat capacity than oil? Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree celsius (or. It requires more energy to raise its temperature by the same amount. A) the highest specific heat capacity? Based on the.
Worksheet Introduction To Specific Heat Capacities Printable And
It requires more energy to raise its temperature by the same amount. Based on the definition above, which of the 4 substances do you think has: Water (see < raph) b). A) the highest specific heat capacity? Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree.
Specific Heat Capacity Worksheet Q=mcΔT Calculations
Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree celsius (or. It requires more energy to raise its temperature by the same amount. A) the highest specific heat capacity? Water (see < raph) b). Based on the definition above, which of the 4 substances do you.
Worksheet Introduction To Specific Heat Capacities Printable And
The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. A) the highest specific heat capacity? Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree celsius (or. Water (see < raph).
Specific Heat Worksheets WorksheetsGO
Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree celsius (or. Based on the definition above, which of the 4 substances do you think has: It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher.
Specific Heat and Heat Capacity Worksheet DocsLib
Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree celsius (or. The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. A) the highest specific heat capacity? Water (see < raph).
Specific Heat Capacity Worksheet
Based on the definition above, which of the 4 substances do you think has: What does it mean if water has a higher specific heat capacity than oil? It requires more energy to raise its temperature by the same amount. Water (see < raph) b). The specific heat capacity of a substance is the energy required to raise the temperature.
Worksheet Introduction To Specific Heat Capacities Printable Word
Based on the definition above, which of the 4 substances do you think has: Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree celsius (or. What does it mean if water has a higher specific heat capacity than oil? Water (see < raph) b). It requires.
Specific Heat Capacity Worksheet With Answers
It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher specific heat capacity than oil? The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius. Based on the definition above, which of the 4.
Specific Heat Capacity Required Practical Worksheet Teaching Resources
It requires more energy to raise its temperature by the same amount. Water (see < raph) b). Based on the definition above, which of the 4 substances do you think has: Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree celsius (or. What does it mean.
A) The Highest Specific Heat Capacity?
Specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree celsius (or. Water (see < raph) b). It requires more energy to raise its temperature by the same amount. What does it mean if water has a higher specific heat capacity than oil?
Based On The Definition Above, Which Of The 4 Substances Do You Think Has:
The specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one degree celsius.