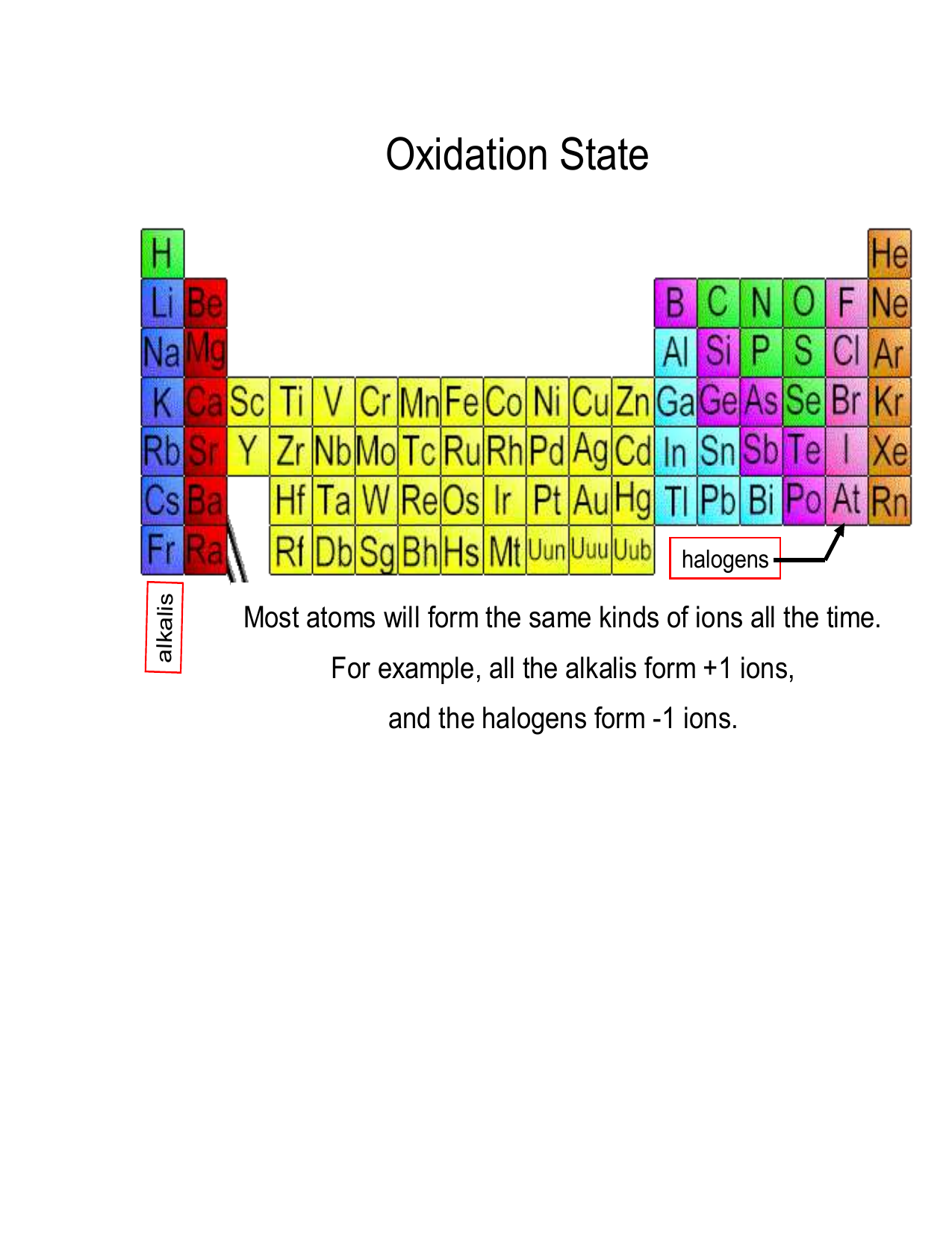
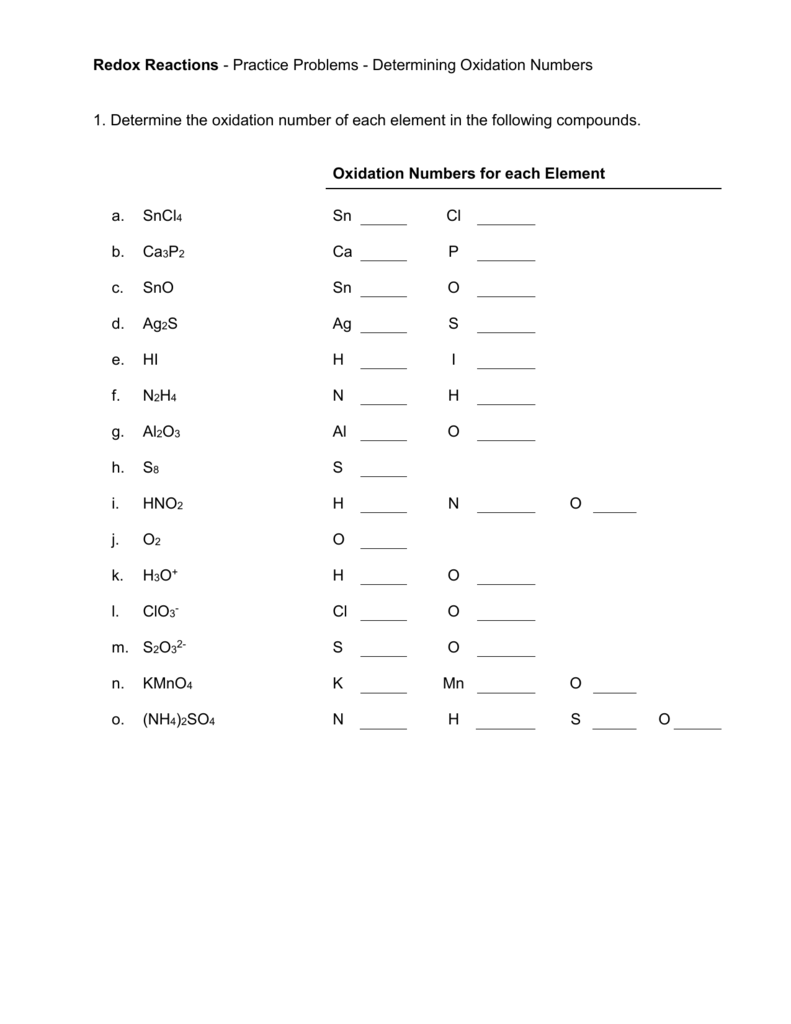
Worksheet Oxidation Numbers - A pure element has an oxidation number of 0. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. 2) the oxidation number of a monatomic ion. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. This exercise is designed to teach the student how to assign oxidation numbers. In the following questions, give the oxidation number of the indicated atoms/ion. Use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these. The oxidation number of an element in a monatomic ion equals the. Rules for assigning oxidation numbers 1. The oxidation number of any uncombined element is 0.
Rules for assigning oxidation numbers 1. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. The oxidation number of any uncombined element is 0. Use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these. Oxidation numbers are very important. 2) the oxidation number of a monatomic ion. The oxidation number of a monatomic ion. In the following questions, give the oxidation number of the indicated atoms/ion. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. The oxidation number of an element in a monatomic ion equals the.
2) the oxidation number of a monatomic ion. A pure element has an oxidation number of 0. The oxidation number of a monatomic ion. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. Rules for assigning oxidation numbers 1. This exercise is designed to teach the student how to assign oxidation numbers. The oxidation number of an element in a monatomic ion equals the. Use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these. Oxidation numbers are very important. The oxidation number of any uncombined element is 0.
Worksheet Assigning Oxidation Numbers Key.doc
In the following questions, give the oxidation number of the indicated atoms/ion. The oxidation number of any uncombined element is 0. Oxidation numbers are very important. A pure element has an oxidation number of 0. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules.
Worksheet 2 Oxidation Numbers Worksheet 2 —
The oxidation number of any uncombined element is 0. The oxidation number of a monatomic ion. Rules for assigning oxidation numbers 1. Oxidation numbers are very important. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules.
Worksheet Oxidation Numbers Give Oxidation Numbers Of All The
This exercise is designed to teach the student how to assign oxidation numbers. Rules for assigning oxidation numbers 1. 2) the oxidation number of a monatomic ion. The oxidation number of any uncombined element is 0. A pure element has an oxidation number of 0.
Worksheet Oxidation Numbers Give Oxidation Numbers Of All The
Oxidation numbers are very important. The oxidation number of a monatomic ion. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. The oxidation number of any uncombined element is 0. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0.
Assigning Oxidation Numbers Worksheet 1 Free Worksheets Printable
The oxidation number of a monatomic ion. Use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. 2) the oxidation number of a monatomic ion. This exercise is designed.
Free Collection of Assigning Oxidation Numbers Worksheets
Rules for assigning oxidation numbers 1. Use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. In the following questions, give the oxidation number of the indicated atoms/ion. The oxidation number of an element in a monatomic.
07 Finding Oxidation Numbers Worksheet
Use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these. The oxidation number of a monatomic ion. The oxidation number of an element in a monatomic ion equals the. In the following questions, give the oxidation number of the indicated atoms/ion. Rules for assigning oxidation numbers 1) the oxidation number of any.
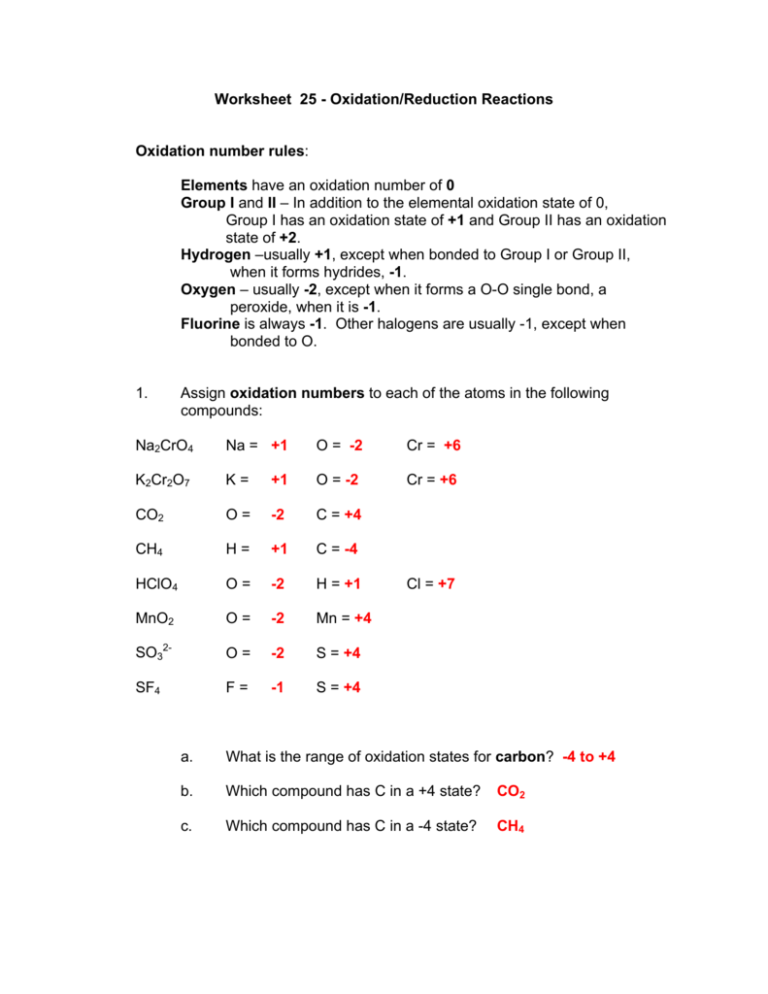
Worksheet 25 Oxidation/Reduction Reactions Oxidation number
This exercise is designed to teach the student how to assign oxidation numbers. The oxidation number of an element in a monatomic ion equals the. The oxidation number of a monatomic ion. 2) the oxidation number of a monatomic ion. A pure element has an oxidation number of 0.
ASSIGNING OXIDATION NUMBERS WORKSHEET
This exercise is designed to teach the student how to assign oxidation numbers. 2) the oxidation number of a monatomic ion. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. The oxidation number of a monatomic ion. Oxidation numbers are very important.
Oxidation Numbers Worksheet
Use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these. The oxidation number of an element in a monatomic ion equals the. Rules for assigning oxidation numbers 1. In the following questions, give the oxidation number of the indicated atoms/ion. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined.
Oxidation Numbers Are Very Important.
Use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these. The oxidation number of a monatomic ion. Rules for assigning oxidation numbers 1. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules.
The Oxidation Number Of An Element In A Monatomic Ion Equals The.
A pure element has an oxidation number of 0. This exercise is designed to teach the student how to assign oxidation numbers. 2) the oxidation number of a monatomic ion. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0.
The Oxidation Number Of Any Uncombined Element Is 0.
In the following questions, give the oxidation number of the indicated atoms/ion.